2-methyl-1 3-butadiene Reacts With Hbr
Reactions of Alkenes |
|---|
Improver Reactions of Alkenes
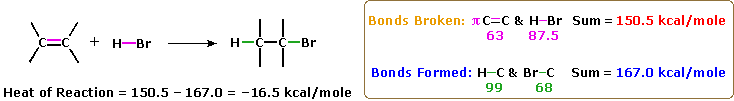
The most mutual chemical transformation of a carbon-carbon double bail is the addition reaction. A big number of reagents, both inorganic and organic, accept been found to add to this functional group, and in this section nosotros shall review many of these reactions. A bulk of these reactions are exothermic, due to the fact that the C-C pi-bond is relatively weak (ca. 63 kcal/mole) relative to the sigma-bonds formed to the atoms or groups of the reagent. Remember, the bail energies of a molecule are the energies required to pause (homolytically) all the covalent bonds in the molecule. Consequently, if the bond energies of the product molecules are greater than the bond energies of the reactants, the reaction volition be exothermic. The following calculations for the addition of H-Br are typical. Note that past convention exothermic reactions have a negative estrus of reaction.
ane. Addition of Potent Brønsted Acids
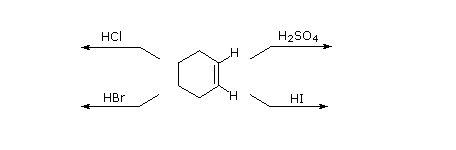
As illustrated by the preceding full general equation, potent Brønsted acids such as HCl, HBr, Hullo & H2And then4, rapidly add together to the C=C functional group of alkenes to give products in which new covalent bonds are formed to hydrogen and to the conjugate base of the acrid. Using the higher up equation every bit a guide, write the addition products expected on reacting each of these reagents with cyclohexene.
 |
Weak Brønsted acids such as h2o (pKa = xv.7) and acerb acrid (pKa = iv.75) exercise not normally add together to alkenes. However, the addition of a stiff acid serves to catalyze the addition of water, and in this way alcohols may be prepared from alkenes. For case, if sulfuric acid is dissolved in water information technology is completely ionized to the hydronium ion, H3O(+), and this strongly acidic (pKa = -1.74) species effects hydration of ethene and other alkenes.
CH2=CH2 + H3O (+) —— > HCH2–CH2 OH + H (+)
The importance of choosing an appropriate solvent for these addition reactions should now be articulate. If the addition of HCl, HBr or Howdy is desired, h2o and alcohols should not exist used. These strong acids will ionize in such solvents to give ROH2 (+) and the nucleophilic oxygen of the solvent volition compete with the halide anions in the final footstep, giving alcohol and ether products. By using inert solvents such as hexane, benzene and methylene chloride, these competing solvent additions are avoided. Considering these additions proceed by way of polar or ionic intermediates, the charge per unit of reaction is greater in polar solvents, such every bit nitromethane and acetonitrile, than in non-polar solvents, such every bit cyclohexane and carbon tetrachloride.
Regioselectivity and the Markovnikov Rule
Only one production is possible from the improver of these strong acids to symmetrical alkenes such as ethene and cyclohexene. However, if the double bond carbon atoms are not structurally equivalent, as in molecules of 1-butene, ii-methyl-2-butene and 1-methylcyclohexene, the reagent conceivably may add together in two different ways. This is shown for 2-methyl-2-butene in the following equation.
| (CH3)2C=CHCHthree + H-Cl |  | (CH3)2CH–CHClCH3 | or | (CH3)2CCl–CHHCH3 |
| two-methyl-two-butene | 2-chloro-three-methylbutane | two-chloro-two-methylbutane |
When improver reactions to such unsymmetrical alkenes are carried out, we find that 1 of the 2 possible constitutionally isomeric products is formed preferentially. Selectivity of this sort is termed regioselectivity. In the above case, 2-chloro-2-methylbutane is near the exclusive product. Similarly, i-butene forms 2-bromobutane as the predominant production on treatment with HBr.

After studying many addition reactions of this kind, the Russian chemist Vladimir Markovnikov noticed a trend in the structure of the favored add-on product. He formulated this trend as an empirical rule nosotros at present call The Markovnikov Rule: When a Brønsted acid, HX, adds to an unsymmetrically substituted double bond, the acidic hydrogen of the acid bonds to that carbon of the double bail that has the greater number of hydrogen atoms already attached to it.
In more homelier vernacular this rule may be restated as, "Them that has gits."
| It is a helpful do to predict the favored product in examples such as those shown below: | |
|---|---|
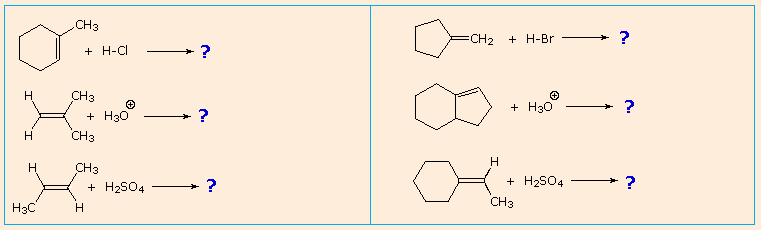 |
Empirical rules similar the Markovnikov Rule are useful aids for remembering and predicting experimental results. Indeed, empirical rules are frequently the get-go step toward practical mastery of a subject, but they seldom constitute true understanding. The Markovnikov Dominion, for case, suggests there are common and important principles at work in these add-on reactions, but it does non tell us what they are. The next step in achieving an understanding of this reaction must be to construct a rational mechanistic model that tin exist tested by experiment.
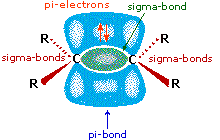 All the reagents discussed here are strong Brønsted acids so, as a first step, it seems sensible to find a base with which the acid tin react. Since we know that these acids practice non react with alkanes, it must be the pi-electrons of the alkene double bond that serve as the base of operations. As shown in the diagram on the right, the pi-orbital extends into the infinite immediately in a higher place and below the airplane of the double bond, and the electrons occupying this orbital may be attracted to the proton of a Brønsted acid. The resulting acid-base equilibrium generates a carbocation intermediate (the conjugate acid of the alkene) which then combines rapidly with the anionic conjugate base of the Brønsted acid. This two-stride mechanism is illustrated for the reaction of ethene with hydrogen chloride by the post-obit equations.
All the reagents discussed here are strong Brønsted acids so, as a first step, it seems sensible to find a base with which the acid tin react. Since we know that these acids practice non react with alkanes, it must be the pi-electrons of the alkene double bond that serve as the base of operations. As shown in the diagram on the right, the pi-orbital extends into the infinite immediately in a higher place and below the airplane of the double bond, and the electrons occupying this orbital may be attracted to the proton of a Brønsted acid. The resulting acid-base equilibrium generates a carbocation intermediate (the conjugate acid of the alkene) which then combines rapidly with the anionic conjugate base of the Brønsted acid. This two-stride mechanism is illustrated for the reaction of ethene with hydrogen chloride by the post-obit equations.
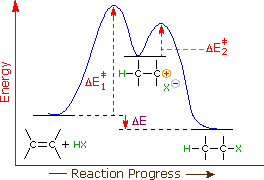 An energy diagram for this two-step addition mechanism is shown to the left. From this diagram nosotros see that the slow or rate-determining step (the first step) is also the product determining step (the anion will necessarily bond to the carbocation site). Electron donating double bond substituents increase the reactivity of an alkene, as evidenced by the increased rate of hydration of 2-methylpropene (two alkyl groups) compared with ane-butene (one alkyl group). Plainly, alkyl substituents deed to increase the rate of addition by lowering the activation energy, ΔE‡ 1 of the rate determining pace, and it is here we should look for a rationalization of Markovnikov'south rule.
An energy diagram for this two-step addition mechanism is shown to the left. From this diagram nosotros see that the slow or rate-determining step (the first step) is also the product determining step (the anion will necessarily bond to the carbocation site). Electron donating double bond substituents increase the reactivity of an alkene, as evidenced by the increased rate of hydration of 2-methylpropene (two alkyl groups) compared with ane-butene (one alkyl group). Plainly, alkyl substituents deed to increase the rate of addition by lowering the activation energy, ΔE‡ 1 of the rate determining pace, and it is here we should look for a rationalization of Markovnikov'south rule.
As expected, electron withdrawing substituents, such as fluorine or chlorine, reduce the reactivity of an alkene to addition past acids (vinyl chloride is less reactive than ethene).
 | Free energy |
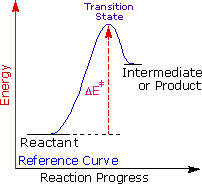
George Hammond formulated a useful principle that relates the nature of a transition state to its location on the reaction path. This Hammond Postulate states that a transition land will be structurally and energetically similar to the species (reactant, intermediate or product) nearest to it on the reaction path. In strongly exothermic reactions the transition state will resemble the reactant species. In strongly endothermic conversions, such as that shown to the correct, the transition state volition resemble the high-energy intermediate or product, and volition rails the energy of this intermediate if information technology changes. This change in transition state free energy and activation energy every bit the stability of the intermediate changes may exist observed by clicking the higher or lower buttons to the correct of the free energy diagram. Three examples may exist examined, and the reference bend is changed to gray in the diagrams for college (magenta) and lower (green) energy intermediates.
The carbocation intermediate formed in the first step of the add-on reaction now assumes a fundamental role, in that information technology direct influences the activation free energy for this step. Independent research shows that the stability of carbocations varies with the nature of substituents, in a fashion similar to that seen for alkyl radicals. The infrequent stability of allyl and benzyl cations is the consequence of charge delocalization, and the stabilizing influence of alkyl substituents, although less pronounced, has been interpreted in a similar fashion.
| Carbocation Stability | CH3 (+) | < | CHiiiCHii (+) | < | (CH3)twoCH(+) | ≈ | CH2=CH-CH2 (+) | < | Chalf-dozenH5CH2 (+) | ≈ | (CHiii)3C(+) |
|---|
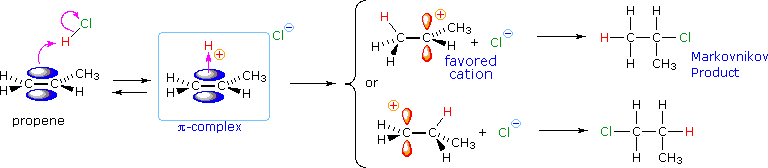
From this information, applying the Hammond Postulate, we make it at a plausible rationalization of Markovnikov'southward rule. When an unsymmetrically substituted double bond is protonated, we expect the more stable carbocation intermediate to be formed faster than the less stable alternative, because the activation free energy of the path to the one-time is the lower of the two possibilities. This is illustrated by the post-obit equation for the addition of hydrogen chloride to propene. Note that the initial acrid-base equilibrium leads to a pi-complex which immediately reorganizes to a sigma-bonded carbocation intermediate. The more stable 2º-carbocation is formed preferentially, and the conjugate base of the Brønsted acid (chloride anion in the case shown below) and then chop-chop bonds to this electrophilic intermediate to form the final production.
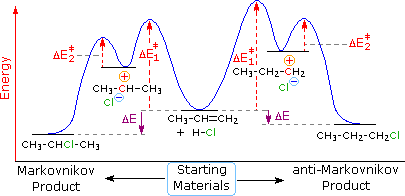
The following energy diagram summarizes these features. Annotation that the pi-circuitous is non shown, since this rapidly and reversibly formed species is mutual to both possible reaction paths.
| A more than extensive discussion of the factors that influence carbocation stability may be accessed by Clicking Hither. |
|---|
2. Rearrangement of Carbocations
The germination of carbocations is sometimes accompanied past a structural rearrangement. Such rearrangements have place by a shift of a neighboring alkyl group or hydrogen, and are favored when the rearranged carbocation is more stable than the initial cation. The add-on of HCl to three,3-dimethyl-one-butene, for case, leads to an unexpected product, 2-chloro-2,3-dimethylbutane, in somewhat greater yield than iii-chloro-2,two-dimethylbutane, the expected Markovnikov production. This surprising result may be explained by a carbocation rearrangement of the initially formed 2º-carbocation to a 3º-carbocation by a ane,2-shift of a methyl grouping. To see this rearrangement click the "Evidence Mechanism" button to the correct of the equation.
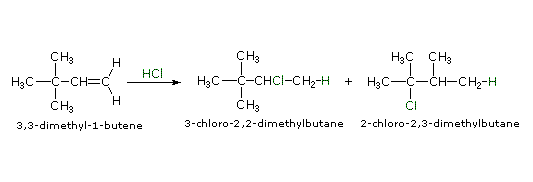 |
Another factor that may induce rearrangement of carbocation intermediates is strain. The addition of HCl to α-pinene, the major hydrocarbon component of turpentine, gives the rearranged production, bornyl chloride, in high yield. As shown in the post-obit equation, this rearrangement converts a 3º-carbocation to a 2º-carbocation, a transformation that is normally unfavorable. Nevertheless, the rearrangement likewise expands a strained four-membered ring to a much less-strained 5-membered ring, and this relief of strain provides a driving force for the rearrangement. A three-dimensional projection view of the rearrangement may exist seen by clicking the "Other View" button. The atom numbers (colored red) for the pinene structure are retained throughout the rearrangement to help orient the viewer. The green numbers in the final product represent the proper numbering of this bicyclic ring system.
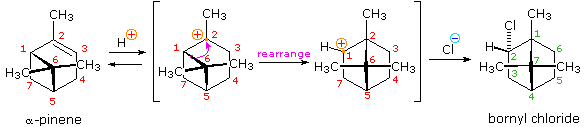 |
The propensity for structural rearrangement shown by certain molecular constitutions, as illustrated in a higher place, serves as a useful probe for the intermediacy of carbocations in a reaction. Nosotros shall apply this exam later.
| An extensive and more detailed discussion of cation induced rearrangements may be accessed by Clicking Here. |
|---|
![]()
3. Add-on of Lewis Acids (Electrophilic Reagents)
The proton is not the only electrophilic species that initiates addition reactions to the double bond. Lewis acids like the halogens, boron hydrides and sure transition metal ions are able to bail to the alkene pi-electrons, and the resulting complexes rearrange or are attacked by nucleophiles to give addition products. The electrophilic character of the halogens is well known. Although fluorine is uncontrollably reactive, chlorine, bromine and to a lesser degree iodine react selectively with the double bail of alkenes. The add-on of chlorine and bromine to alkenes, every bit shown in the following full general equation, gain by an initial electrophilic assault on the pi-electrons of the double bond. Iodine adds reversibly to double bonds, merely the equilibrium does not normally favor the addition production, and then it is not a useful preparative method. Dihalo-compounds in which the halogens are juxtaposed in the manner shown are chosen vicinal, from the Latin vicinalis, meaning neighboring.
Other halogen containing reagents which add to double bonds include hypohalous acids, HOX, and sulfenyl chlorides, RSCl. These reagents are unsymmetrical, then their addition to unsymmetrical double bonds may in principle take place in two ways. In practice, these addition reactions are regioselective, with one of the two possible constitutionally isomeric products beingness favored. The electrophilic moiety of these reagents is the halogen.
(CH3)iiC=CH2 + HOBr —— > (CHiii)iiCOH-CHtwo Br
(CH3)2C=CH2 + CviH5 SCl —— > (CHiii)2CCl-CHtwo SC6H5
The regioselectivity of the above reactions may be explained by the aforementioned machinery we used to rationalize the Markovnikov rule. Thus, bonding of an electrophilic species to the double bail of an alkene should effect in preferential germination of the more stable (more highly substituted) carbocation, and this intermediate should then combine rapidly with a nucleophilic species to produce the addition product. This is illustrated by the following equation.
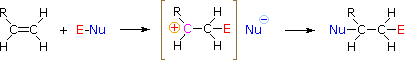
To apply this mechanism we need to determine the electrophilic moiety in each of the reagents. 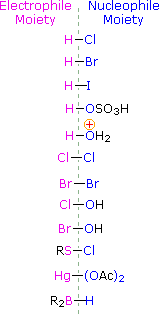 Past using electronegativity differences nosotros can dissect common addition reagents into electrophilic and nucleophilic moieties, as shown on the correct. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's ca. 8) do not react equally proton donors; and since oxygen is more than electronegative than chlorine or bromine, the electrophile will exist a halide cation. The nucleophilic species that bonds to the intermediate carbocation is then hydroxide ion, or more likely water (the usual solvent for these reagents), and the products are chosen halohydrins. Sulfenyl chlorides add together in the contrary style because the electrophile is a sulfur cation, RS(+), whereas the nucleophilic moiety is chloride anion (chlorine is more electronegative than sulfur).
Past using electronegativity differences nosotros can dissect common addition reagents into electrophilic and nucleophilic moieties, as shown on the correct. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's ca. 8) do not react equally proton donors; and since oxygen is more than electronegative than chlorine or bromine, the electrophile will exist a halide cation. The nucleophilic species that bonds to the intermediate carbocation is then hydroxide ion, or more likely water (the usual solvent for these reagents), and the products are chosen halohydrins. Sulfenyl chlorides add together in the contrary style because the electrophile is a sulfur cation, RS(+), whereas the nucleophilic moiety is chloride anion (chlorine is more electronegative than sulfur).
| If you understand this machinery you should exist able to write products for the following reactions: | |
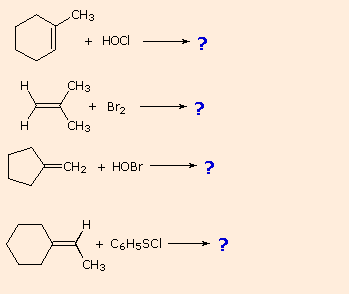 | |
The addition products formed in reactions of alkenes with mercuric acetate and boron hydrides (compounds shown at the bottom of of the reagent list) are normally not isolated, just instead are converted to alcohols by a substitution reaction. These important synthetic transformations are illustrated for 2-methylpropene by the following equations, in which the electrophilic moiety is colored cherry-red and the nucleophile bluish. The meridian reaction sequence illustrates the oxymercuration process and the bottom is an example of hydroboration.
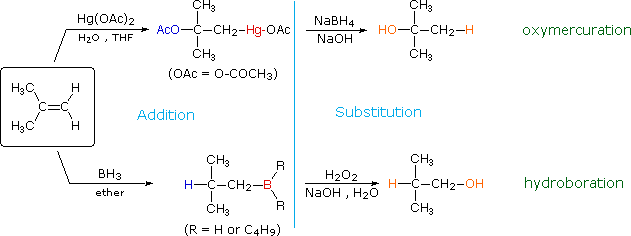
The lite bluish vertical line separates the improver reaction on the left from the substitution on the correct. The atoms or groups that take been added to the original double bond are colored orange in the final production. In both cases the overall reaction is the addition of h2o to the double bail, but the regioselectivity is reversed. The oxymercuration reaction gives the product predicted past Markovnikov'south dominion; hydroboration on the other paw gives the "anti-Markovnikov" production. Complementary reactions such as these are important because they permit us to direct a molecular transformation whichever style is desired.
Mercury and boron are removed from the organic substrate in the second step of oxymercuration and hydroboration respectively. These reactions are seldom discussed in particular; withal, it is worth noting that the mercury moiety is reduced to metallic mercury past borohydride (probably by way of radical intermediates), and boron is oxidized to borate by the alkaline peroxide. Addition of hydroperoxide anion to the electrophilic borane generates a tetra-coordinate boron peroxide, having the general formula RiiiB-O-OH(-). This undergoes successive intramolecular shifts of alkyl groups from boron to oxygen, accompanied in each issue by boosted peroxide addition to electron deficient boron. The retention of configuration of the migrating alkyl grouping is attributed to the intramolecular nature of the rearrangement.
Since the oxymercuration sequence gives the same hydration product as acid-catalyzed addition of water (see Brønsted acid addition), we might question why this ii-step process is used at all. The reason lies in the milder reaction conditions used for oxymercuration. The stiff acid used for direct hydration may not exist tolerated by other functional groups, and in some cases may cause molecular rearrangement (see above).
The addition of borane, BH3, requires additional comment. In pure form this reagent is a dimeric gas B2Hvi, called diborane, but in ether or THF solution information technology is dissociated into a solvent coordinated monomer, R2O-BHthree. Although diborane itself does not react hands with alkene double bonds, H.C. Brown (Purdue, Nobel Prize 1979) discovered that the solvated monomer adds speedily nether mild conditions. Boron and hydrogen accept rather similar electronegativities, with hydrogen being slightly greater, and then it is not likely at that place is significant dipolar graphic symbol to the B-H bond. Since boron is electron deficient (it does not have a valence shell electron octet) the reagent itself is a Lewis acrid and tin can bond to the pi-electrons of a double bond by displacement of the ether moiety from the solvated monomer. As shown in the following equation, this bonding might generate a dipolar intermediate consisting of a negatively-charged boron and a carbocation. Such a species would not be stable and would rearrange to a neutral production by the shift of a hydride to the carbocation middle. Indeed, this hydride shift is believed to occur concurrently with the initial bonding to boron, as shown past the transition state drawn beneath the equation, so the discrete intermediate shown in the equation is non actually formed. Nevertheless, the carbocation stability rule cited above remains a useful mode to predict the products from hydroboration reactions. You may right the top equation by clicking the push on its right. Note that this addition is unique among those we have discussed, in that it is a single-step process. Also, all three hydrogens in borane are potentially reactive, and so that the alkyl borane product from the first improver may serve as the hydroboration reagent for two additional alkene molecules.
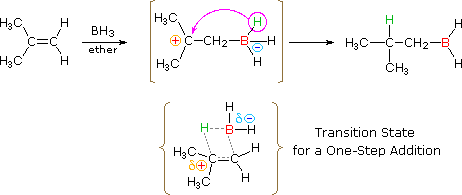 | |
To examine models of B2H6. and its dissociation in THF
![]()
Stereoselectivity in Addition Reactions to Double Bonds
As illustrated in the drawing on the correct, the pi-bond fixes the carbon-carbon double bond in a planar configuration, and does not permit free rotation about the double bond itself.  We see then that addition reactions to this function might occur in 3 different ways, depending on the relative orientation of the atoms or groups that add to the carbons of the double bond: (i) they may bond from the aforementioned side, (two) they may bond from opposite sides, or (iii) they may bond randomly from both sides. The first two possibilities are examples of stereoselectivity, the first being termed syn-addition, and the 2nd anti-addition. Since initial electrophilic assault on the double bond may occur equally well from either side, information technology is in the second footstep (or stage) of the reaction (bonding of the nucleophile) that stereoselectivity may be imposed.
We see then that addition reactions to this function might occur in 3 different ways, depending on the relative orientation of the atoms or groups that add to the carbons of the double bond: (i) they may bond from the aforementioned side, (two) they may bond from opposite sides, or (iii) they may bond randomly from both sides. The first two possibilities are examples of stereoselectivity, the first being termed syn-addition, and the 2nd anti-addition. Since initial electrophilic assault on the double bond may occur equally well from either side, information technology is in the second footstep (or stage) of the reaction (bonding of the nucleophile) that stereoselectivity may be imposed.
If the 2-pace mechanism described higher up is right, and if the carbocation intermediate is sufficiently long-lived to freely-rotate nearly the sigma-bail component of the original double bond, we would expect to notice random or not-stereoselective addition in the products. On the other paw, if the intermediate is short-lived and factors such every bit steric hindrance or neighboring grouping interactions favor one side in the second step, then stereoselectivity in production formation is likely. The following tabular array summarizes the results obtained from many studies, the formula HX refers to all the strong Brønsted acids. The interesting differences in stereoselectivity noted here provide further insight into the mechanisms of these addition reactions.
| Reagent | H–X | Xtwo | HO–X | RS–Cl | Hg(OAc)2 | BH3 |
|---|---|---|---|---|---|---|
| Stereoselectivity | mixed | anti | anti | anti | anti | syn |
ane. Brønsted Acid Additions
The stereoselectivity of Brønsted acid addition is sensitive to experimental conditions such equally temperature and reagent concentration. The selectivity is ofttimes anti, merely reports of syn selectivity and not-selectivity are not uncommon. Of all the reagents discussed here, these stiff acrid additions (E = H in the following equation) come closest to proceeding by the proposed two-step mechanism in which a discrete carbocation intermediate is generated in the first step. Such reactions are most prone to rearrangement when this is favored past the alkene construction.

2. Addition Reactions Initiated past Electrophilic Element of group vii
The halogens chlorine and bromine add chop-chop to a wide diverseness of alkenes without inducing the kinds of structural rearrangements noted for strong acids (get-go example below). The stereoselectivity of these additions is strongly anti, as shown in many of the post-obit examples.
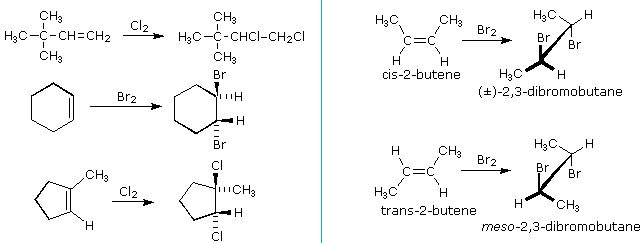
An important principle should be restated at this time. The alkenes shown here are all achiral, simply the improver products have chiral centers, and in many cases may be equally enantiomeric stereoisomers. In the absence of chiral catalysts or reagents, reactions of this kind will always give racemic mixtures if the products are enantiomeric. On the other manus, if two chiral centers are formed in the addition the reaction will be diastereomer selective. This is clearly shown by the addition of bromine to the isomeric 2-butenes. Anti-improver to cis-2-butene gives the racemic product, whereas anti-addition to the trans-isomer gives the meso-diastereomer.
We tin can account both for the high stereoselectivity and the lack of rearrangement in these reactions past proposing a stabilizing interaction between the developing carbocation centre and the electron rich halogen atom on the next carbon. This interaction, which is depicted for bromine in the following equation, delocalizes the positive accuse on the intermediate and blocks halide ion attack from the syn-location.
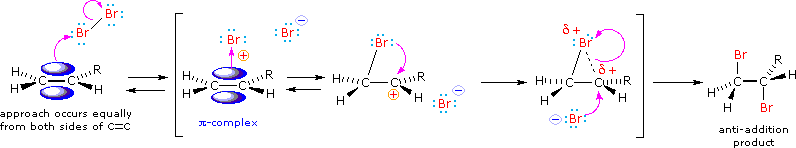
The stabilization provided by this halogen-carbocation bonding makes rearrangement unlikely, and in a few cases three-membered cyclic halonium cations take been isolated and identified as truthful intermediates. A resonance description of such a bromonium ion intermediate is shown beneath. The positive accuse is delocalized over all the atoms of the ring, but should be concentrated at the more substituted carbon (carbocation stability), and this is the site to which the nucleophile will bond.
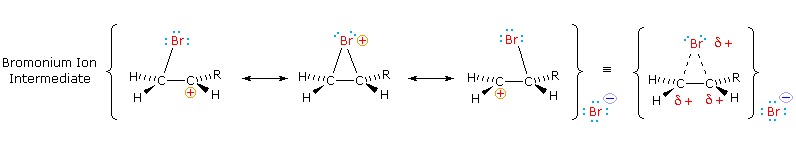
| The stereoselectivity described here is in large part due to a stereoelectronic effect. |
|---|
Because they go on past mode of polar ion-pair intermediates, chlorine and bromine add-on reactions are faster in polar solvents than in not-polar solvents, such every bit hexane or carbon tetrachloride. Yet, in guild to prevent solvent nucleophiles from competing with the halide anion, these not-polar solvents are oft selected for these reactions. In water or alcohol solution the nucleophilic solvent may open up the bromonium ion intermediate to give an α-halo-alcohol or ether, together with the expected vic-dihalide. Such reactions are sensitive to pH and other factors, so when these products are desired it is necessary to modify the addition reagent. Aqueous chlorine exists as the following equilibrium, Keq ≈ x-4. By adding AgOH, the concentration of HOCl can be greatly increased, and the chlorohydrin improver production obtained from alkenes.
| Cl2 + H2O |  | HOCl + HCl |
The more widely used HOBr reagent, hypobromous acid, is unremarkably fabricated by hydrolysis of North-bromoacetamide, every bit shown below. Both HOCl and HOBr additions occur in an anti fashion, and with the regioselectivity predicted by this machinery (OH bonds to the more substituted carbon of the alkene).
| CH3CONHBr + H2O |  | HOBr + CHthreeCONHii |
Vicinal halohydrins provide an alternative route for the epoxidation of alkenes over that of reaction with peracids. As illustrated in the following diagram, a base induced intramolecular exchange reaction forms a three-membered cyclic ether called an epoxide. Both the halohydrin formation and halide displacement reactions are stereospecific, then stereoisomerism in the alkene will be reflected in the epoxide product (i.e. trans-2-butene forms a trans-disubstituted epoxide). A general procedure for forming these useful compounds will be discussed in the next section.
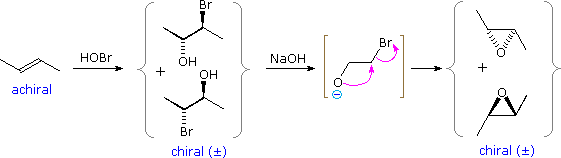
| Other Addition Reactions |
|---|
This page is the property of William Reusch. Comments, questions and errors should exist sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in capacity building in chemic education. 05/05/2013
2-methyl-1 3-butadiene Reacts With Hbr,
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/addene1.htm
Posted by: rubioalwass.blogspot.com


0 Response to "2-methyl-1 3-butadiene Reacts With Hbr"
Post a Comment